Trust your manufactured products to the premier name in ultra-cold storage.
Trust your manufactured drug products with the premier name in ultra-cold storage.
Our capabilities range from storage and cold chain logistics management to pharmaceutical distribution of drug products in their journey from development to commercialization.
Azenta Life Sciences understands the challenges that drug manufacturers like pharmaceutical companies and CDMOs face - starting with ultra-cold temperature environments. Manufactured drug products have rigorous cold storage standards, with specific needs, which are critical for the efficacy and shelf-life of temperature sensitive drugs. Adherence to these requirements can be very challenging, especially when the products need to be transported from/to various points-of-use. Our experts specialize in cGMP (Current Good Manufacturing Practice) controlled cold chain storage as well as cold chain management to help safeguard the integrity and quality of your indispensable products.
From ambient temperature to our core competency in ULTRA-COLD STORAGE - we maintain secure cold chain logistics, every step of the way.
Pharmaceutical Storage Temperature Capabilities Types of Materials Stored
- Ambient
- Refrigerated
Freezer (-20°C to -30°C)
- Ultra-low freezer (-40°C to -80°C)
- 液态氮(-150°C到-196°C)
- Active Pharmaceutical Ingredients (API)
- Bulk drug substances
- Finished drug products (investigational medicinal drugs and commercialized)
- Retains

ULTRA-COLD STORAGECold and ultra-cold storage of drugs from investigational medicinal products to commercialized drug products - retains, vaccines, cell lines, mRNA, and other therapeutic materials are maintained in our fully licensed and accredited cGMP warehouses. |
|
COLD CHAIN LOGISTICSMaintaining the integrity and quality of active pharmaceutical ingredients (API) to finished drug products needing exact temperature requirements during transport is our specialty. Our118bet金博宝 cold chain logistics solutions include cold chain transportation of bulk shipments in our fleet of mobile biorepositories or arranging shipments to/from our global cGMP facilities. |
|
INVENTORY MANAGEMENTUsers access material data through asecure 21 CFR, Part 11 compliant software, providing real-time visibility to inventory and complete audit trail. |
在温度偏差可以显著affect a drug's efficacy, cost companies a substantial amount of money, and delay bringing your drug to market. When small to large scale CDMOs, pharma, and biotech companies demand ultra-cold storage or pharmaceutical distribution services - they turn to Azenta Life Sciences. Our highly-specialized team works with you to develop a comprehensive, custom plan to receive, distribute, and manage your drug materials.
We want to partner with you!
- Over 200,000 sq. ft. dedicated to ultra-cold storage of pharmaceutical products
- EMA and PMDA certified for cGMP storage
- National Association of Boards of Pharmacy and US State Board of Pharmacy License
- GTP licensed by the USA FDA
- Multiple redundancy systems
- Over 150+ unwavering best practices and QMS for regulated storage and logistics
- 21CFR, Part 11 compliant software with 24/7 access to inventory records
- Safe and secure cold chain storage and chain-of-custody tracking
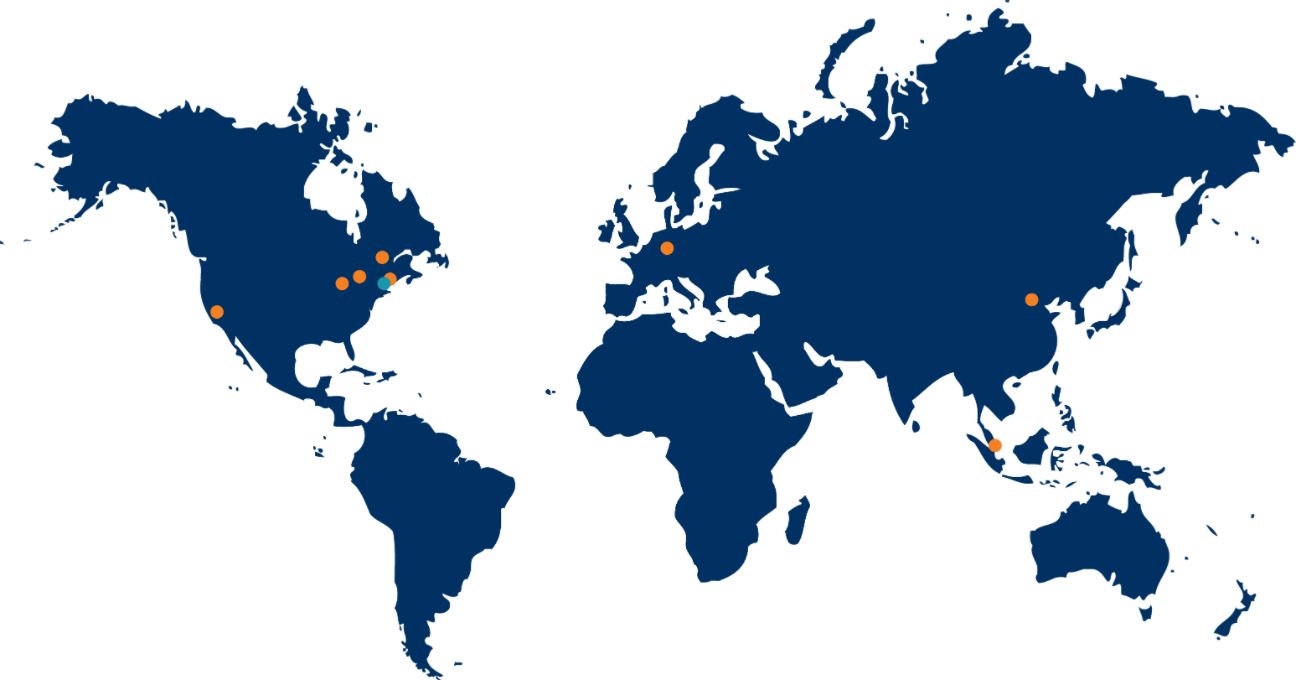
cGMP Storage and Distribution
- Indianapolis, IN, USA
- Griesheim, Germany
cGMP Storage
- Bronx, NY, USA
- 美国克利夫兰哦
- Fresno, CA, USA
- Beijing, China
- Montreal, Canada
- Singapore, Singapore
Support available at customer locations


